- Sp2 Hybridized Carbon
- Sp3 Hybridized Carbons
- Sp2-hybridized Carbon Atoms Benzene
- Double Bond Carbon Sp2 Hybridization
- Sp2 Hybridized Carbons
- Sp2 Hybridized Carbon Ir
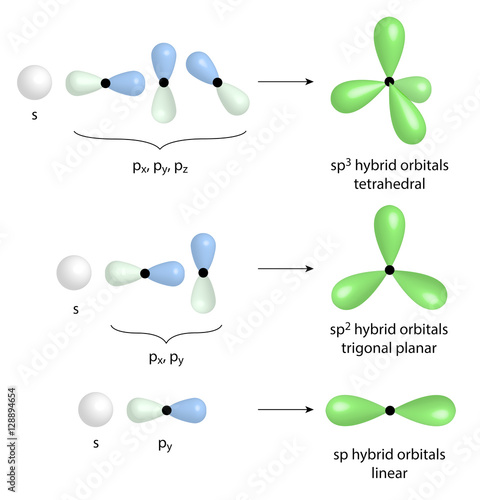
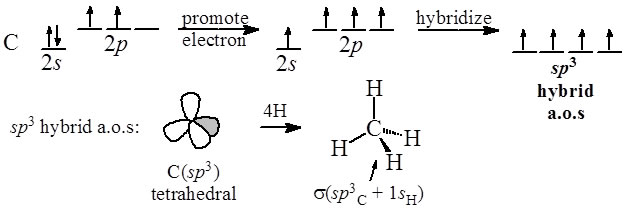
Most importantly we have sp3, sp2 and sp hybridisation. Sp3 Hybridisation in Methane (CH4): The best way I can describe sp3 hybridisation is in Methane (also the most basic choice!). This is simplified for expression. Remember that Carbon has 6 electrons. In methane (CH4), 1 Carbon binds with 4 Hydrogens. The sp 2 hybridization is the mixing of one s and two p atomic orbitals, which involves the promotion of one electron in the s orbital to one of the 2p atomic orbitals. The combination of these atomic orbitals creates three new hybrid orbitals equal in energy-level. Carbon is said to be sp3 hybridised when it it is SINGLY BONDED with any other 3 atomsfor example: mathane (CH4) carbon is said to be sp2 hybridised when it is DOUBLY BONDED with any 1 atom and SINGLY BONDED with any other 2 atoms.for example ethene (CH2=CH2). 5.9K views View 17 Upvoters.
Your organic chemistry course will cover many different types of isomers.
Isomers have the same molecular formula but something about them is different.
Geometric isomers, a type of stereoisomer, differ in their geometry or shape. This happens when substituents are LOCKED in a specific relationship to each other.
I say locked because, unlike conformational isomers in Newman Projections, you can’t simply rotate the molecule to change the relationship between substituents.
In this tutorial, we’ll look at alkene geometric isomers including cis trans and E Z.
Cis/Trans Isomerism
Cis/Trans isomerism is typically seen with substituents on either side of the alkene double bond.
How does this happen?
Alkene double bonds occur between sp2 hybridized carbon atoms. Recall: sp2 hybrids have a trigonal-planar or ‘flat’ geometry.
(Not comfortable with this? Review sp2 Hybridization.)
But it’s not the hybrid we’re looking at.
Instead, it’s the un-hybridized p-orbital that forms a SECOND bond between the 2 carbon atoms.
An sp3 hybridized single or simga bond is free to rotate.
Sp2 pi bonds are locked in place.
Sp2 Hybridized Carbon
The only way to rotate this bond is to break the double bond, rotate, and reform the double bond –which is typically not observed.
In fact, this requires high energy, as you’ll see in your Diels Alder reactions later on. Nexus vst for mac download.
Take a look at the following generic alkene and it’s 4 substituents:
Carbon 1 has substituents A and B; Carbon 2 has substituents C and D.
But notice specifically how A is on the same side as C, and B is on the same side as D.
The only way to bring A next to D is to break the pi bond, rotate the molecule, and reform the pi bond. Otherwise A is locked in place near C, and B is locked in place near D.
Cis vs Trans Alkenes
Let’s take a look at 2 versions of 2-butene: 2-butene is a 4-carbon chain with a double bond between carbons 2 and 3.
So, we can draw this incorrectly, as a linear molecule:
Or, draw each sp2 carbon at a 120 degree bond angle. This gives me the option of placing both methyl groups up, down, or one up and one down.
The first two are actually the same: both cis. You see, I can flip the molecule and make the first superimpose (overlap) the second without breaking any bonds.
The third is unique. The only way to superimpose the third is to break the double bond.
Cis Alkenes
I like to think of cis as ‘sisters’. They are together on the same side.
Cis alkenes have substituents on the same side of the double bond.
Trans Alkenes
I like to think of trans substituents as having ‘transferred away from each other.’ Putting them on opposite sides.
Trans alkenes have their substituents on opposite sides.
Sp3 Hybridized Carbons
Naming Cis/Trans Alkenes:
Once you’ve identified cis/trans alkenes, naming them is fairly simple.
1) First, name the alkene using the tutorial linked below.
2) Then, simply add ‘cis’ or ‘trans’ in front of the name.
Take the 2 geometric isomers of 2-butene:
Their proper names are as follows:
When there is only one pi bond, you don’t have to specify which carbon is cis or trans since. It’s self-understood.
When you have more than one double bond on the molecule, you must specify which is cis and which is trans.
Take this molecule for example: 2,5-octadiene
This molecule has 2 pi bonds. One cis and one trans.
Since there is more than one pi bond, you have to specify which pi bond is cis and which is trans.
Alkene stability
Not all isomers have the same stability.
It’s all about stability – in organic chemistry or science in general.
Trans alkenes are MORE STABLE than their cis counterparts.
This is more apparent with larger substituents.
Trans Alkenes

In a trans alkene, the substituents are facing away from each other.
They don’t ‘get in each other’s faces’ and therefore, don’t mind the other groups.
Cis Alkenes
Cis alkene substituents are close together and will ‘get in each others faces.’ This causes ‘arguments’ when one group invades the other’s personal space.
When the groups try to move away from each other, they cause strain on the molecule.
All of this leads to an unhappy and higher energy cis conformation.
Cis & Trans on Cyclic Compounds
Ring structures or cyclic compounds can also exhibit cis/trans isomerism without the presence of a pi bond.
Remember, substituents will be cis and trans if they are locked in place. Pi bonds are one way to lock them in place. Rings are another matter.
For example, in 1,2-dimethylcyclohexane, I can show both substituents going into the page or both going out of the page.
Since they’re pointing in the same direction, they are cis to each other.
If I show one going into the page and one going out of the page. They are trans to each other.
Even though the carbons are sp3 and sigma bound to each other, the molecule itself cannot rotate because of the ring structure. Locked.
The only way to turn cis-1,2-dimethylcyclohexane into trans-1,2-dimethylcyclohexane, is to break open the ring, rotate, and reform the ring.
What if there’s more than one substituent on the sp2 carbon?
Till now, we’ve looked at molecules with just one substituent on either side of the sp2 pi bound carbon.
What happens if we have a pi bond with 2 different atoms or groups on the sp2 carbon?
Take a look at 3-methyl-2-pentene:
Here in line structure:
You can draw this molecule in 2 different ways. But will you compare the red methyl or red ethyl to the green methyl when choosing cis or trans?
While some professors WILL teach you to compare the larger groups, the answer is that you CANNOT compare simply choose one for cis and trans.
Sp2-hybridized Carbon Atoms Benzene
Introducing the E Z Notation
When a pi bond has more than one substituent on each side, or contains non-carbon substituents, we’ll need a more advanced system for identifying geometric isomerism.
The E Z system requires ranking the groups on either side of the pi bond. We must determine if the higher priority groups are next to each other, Z (think cis), or away from each other, E (think trans).
But first, we have to learn how to rank groups using the Cahn-Ingold-Prelog notation.
The video below is from my chirality series, but teaches this concept in detail. Start watching from 0:52
Cahn-Ingold-Prelog in summary:
We’re ranking atoms based on their atomic number.
Not mass of the group, not size of the group.
The higher the atomic number of the atom directly attached, the higher the priority.
Here are the 10 most common atoms you’ll come across from high to low priority:
I > Br > Cl > S > P > F > O > N > C > H
Here’s My Simple Approach
- Highlight your double bond, then look at just one half of the molecule at a time. Use your hand or another paper to cover the other half of the molecule.
- Determine which group is higher priority and put a number 1.
I like to draw an arrow perpendicular to the pi bond so I can clearly see if it’s up or down by comparison. - Do the same thing for the other side.
E is for Eeposite, Z is for Ze Zame Zide
If the two high priority groups are opposite to each other, think of them as being ‘eeposite’ to each other.
E is for Eeposite.
If the two high priority groups are on the the same side, or should I say on ‘Ze Zame Zide,’ they are Z.
This applies to molecules that have more than just 1 carbon on either side of double bond.
Ze Zame Zide.
Let’s go back to the example above:
On the left, OH outranks ethyl since oxygen has a higher atomic number when compared to carbon. OH is #1 and points down.
On the right, Cl outranks methyl since chlorine has a higher atomic number when compared to carbon. Cl is #1 and points down.
Since both arrows point in the same direction (down), we conclude that the priority groups are on Ze Zame Zide making it Z.
2 Equal Priority Groups
Sometimes you’ll see a trick question where an sp2 carbon will have 2 of the exact same groups.
Since you cannot rank one over the other, there will be NO cis/trans or E/Z isomerism.
Here are 2 common examples:
1) A terminal pi bond
Carbon #1 in 1-butene has 2 hydrogen atoms.
Since H vs H have the same exact priority, this molecule will have no cis/trans or E/Z isomerism.
2) Same exact groups on the same sp2 pi bound carbon.
Carbon #2 in 2-methyl-2-butene has 2 CH3 groups.
One appears to be part of the parent chain, the second appears to be a methyl substituent.
However, when CH3 is compared to CH3 they rank exactly the same.
This molecule will have no cis/trans n/or E/Z isomerism.
Cis and Trans vs E and Z
If we go back to our cis/trans practice problems, such as cis and trans 2-butene, you’ll see that we can use the E/Z system here as well.
Carbon 2 and 3 each have a methyl group outranking a hydrogen atom. When they are cis, you get Z. When they are trans you get E.
A word of caution
You CAN use E/Z for cis/trans isomers, but you cannot use cis/trans for complex E/Z isomers as we’ve already shown above.
In Summary
Cis vs trans and E vs Z isomers are geometric isomers that occur when substituents are locked in position next to or opposite each other. This is seen in both double bonds for alkenes, and substituents on ring structures.
Cis alkenes are on the same size, trans alkenes are on opposite sides. When the substituents are more complicated use the more advanced E/Z notation after determining the relationship of high priority groups.
Objectives
After completing this section, you should be able to
- define aromaticity in terms of the Hückel 4n + 2 rule.
- use the Hückel 4n + 2 rule to determine whether or not a given polyunsaturated cyclic hydrocarbon should exhibit aromatic properties.
- describe the difference in properties between an aromatic hydrocarbon, such as benzene, and a non-aromatic polyunsaturated cyclic hydrocarbon, such as cyclobutadiene or cyclooctatetraene.
- draw molecular orbital diagrams for aromatic species, such as benzene, the cyclopentadienyl anion and pyridine, and compare these diagrams with those obtained for non-aromatic species, such as cyclobutadiene and the cyclopentadienyl cation.
Study Notes
The following mnemonic device will help you establish the approximate energy levels for the molecular orbitals of various organic ring systems.
Whatever the size of the ring, place one point of the ring down to the bottom. The corners of the ring, where the carbons are located, will roughly approximate the location and pattern of the molecular orbital energy levels. Cut the ring exactly in half. The energy levels in the top half will be anti-bonding (Ψ*) orbitals and those in the bottom will be bonding (Ψ) orbitals. If the carbons fall directly in the centre of the ring (e.g., four-membered rings) the energy levels there are non-bonding.
cyclopropenyl ring (three-membered ring)
cyclobutadienyl ring (four-membered ring)
cyclopentadienyl ring (five-membered ring)
In 1931, German chemist and physicist Erich Hückel proposed a theory to help determine if a planar ring molecule would have aromatic properties. His rule states that if a cyclic, planar molecule has 4n+2 π electrons, it is considered aromatic. This rule would come to be known as Hückel's Rule.
Four Criteria for Aromaticity
When deciding if a compound is aromatic, go through the following checklist. If the compound does not meet all the following criteria, it is likely not aromatic.
- The molecule is cyclic (a ring of atoms)
- The molecule is planar (all atoms in the molecule lie in the same plane)
- The molecule is fully conjugated(p orbitals at every atom in the ring)
- The molecule has 4n+2 π electrons (n=0 or any positive integer)
Why 4n+2 π Electrons?
According to Hückel's Molecular Orbital Theory, a compound is particularly stable if all of its bonding molecular orbitals are filled with paired electrons. This is true of aromatic compounds, meaning they are quite stable. With aromatic compounds, 2 electrons fill the lowest energy molecular orbital, and 4 electrons fill each subsequent energy level (the number of subsequent energy levels is denoted by n), leaving all bonding orbitals filled and no anti-bonding orbitals occupied. This gives a total of 4n+2 (pi) electrons. You can see how this works with the molecular orbital diagram for the aromatic compound, benzene, below. Benzene has 6 (pi) electrons. Its first 2 (pi) electrons fill the lowest energy orbital, and it has 4 (pi) electrons remaining. These 4 fill in the orbitals of the succeeding energy level. Notice how all of its bonding orbitals are filled, but none of the anti-bonding orbitals have any electrons.
To apply the 4n+2 rule, first count the number of π electrons in the molecule. Then, set this number equal to 4n+2 and solve for n. If is 0 or any positive integer (1, 2, 3,..), the rule has been met. For example, benzene has six (pi) electrons:
Double Bond Carbon Sp2 Hybridization
[begin{align} 4n + 2 &= 6 4n &= 4 n &= 1 end{align}]
For benzene, we find that (n=1), which is a positive integer, so the rule is met.
How Can You Tell Which Electrons are π Electrons?
Perhaps the toughest part of Hückel's Rule is figuring out which electrons in the compound are actually π electrons. Once this is figured out, the rule is quite straightforward. (pi) electrons lie in p orbitals and (sp^2) hybridized atoms have 1 p orbital each. So if every molecule in the cyclic compound is sp2 hybridized, this means the molecule is fully conjugated (has 1 p orbital at each atom), and the electrons in these p orbitals are the π electrons. A simple way to know if an atom is sp2 hybridized is to see if it has 3 attached atoms and no lone pairs of electrons. This video provides a very nice tutorial on how to determine an atom's hybridization. In a cyclic hydrocarbon compound with alternating single and double bonds, each carbon is attached to 1 hydrogen and 2 other carbons. Therefore, each carbon is sp2 hybridized and has a p orbital. Let's look at our previous example, benzene:
Each double bond (π bond) always contributes 2 π electrons. Benzene has 3 double bonds, so it has 6 π electrons.
Aromatic Ions
Hückel's Rule also applies to ions. As long as a compound has 4n+2 π electrons, it does not matter if the molecule is neutral or has a charge. For example, cyclopentadienyl anion is an aromatic ion. How do we know that it is fully conjugated? That is, how do we know that each atom in this molecule has 1 p orbital? Let's look at the following figure. Carbons 2-5 are sp2 hybridized because they have 3 attached atoms and have no lone electron pairs. What about carbon 1? Another simple rule to determine if an atom is sp2 hybridized is if an atom has 1 or more lone pairs and is attached to an sp2 hybridized atom, then that atom is sp2 hybridized also. This video explains the rule very clearly. Therefore, carbon 1 has a p orbital. Cyclopentadienyl anion has 6 π electrons and fulfills the 4n+2 rule.
Heterocyclic Aromatic Compounds
Sp2 Hybridized Carbons
So far, you have encountered many carbon homocyclic rings, but compounds with elements other than carbon in the ring can also be aromatic, as long as they fulfill the criteria for aromaticity. These molecules are called heterocyclic compounds because they contain 1 or more different atoms other than carbon in the ring. A common example is furan, which contains an oxygen atom. We know that all carbons in furan are sp2 hybridized. But is the oxygen atom sp2 hybridized? The oxygen has at least 1 lone electron pair and is attached to an sp2 hybridized atom, so it is sp2 hybridized as well. Notice how oxygen has 2 lone pairs of electrons. How many of those electrons are π electrons? An sp2 hybridized atom only has 1 p orbital, which can only hold 2 electrons, so we know that 1 electron pair is in the p orbital, while the other pair is in an sp2 orbital. So, only 1 of oxygen's 2 lone electron pairs are π electrons. Furan has 6 π electrons and fulfills the 4n+2 rule.

A Common Misconception
A very common misconception is that hybridization can be used to predict the geometry, or that hybridization somehow involves an energy cost associated with 'promoting' electrons into the hybrid orbitals. This is entirely wrong. Hybridization is always determined by geometry. You can only assign hybridization states to an atom if you already know its geometry, based on some experimental or theoretical evidence. The geometry of the oxygen in furan is trigonal planar and therefore the hybridization must be (sp^2).
The specific rule is that if you have an (sp^2) conjugated system, the lone pair will be involved if it makes the system more stable. In this case, conferring Hückel (4n+2) aromaticity. For furan with two lone pairs on the oxygen atom, if we count electrons from the carbon atoms, we have 4 (one per carbon). So adding two electrons from one of the lone pairs will give 6 = 4(1)+2, so Hückel rule is applicable and furan is aromatic.
Exercise (PageIndex{1})
Using the criteria for aromaticity, determine if the following molecules are aromatic:
- Aromatic - only 1 of S's lone pairs counts as π electrons, so there are 6 π electrons, n=1
- Not aromatic - not fully conjugated, top C is sp3 hybridized
- Not aromatic - top C is sp2 hybridized, but there are 4 π electrons, n=1/2
- Aromatic - N is using its 1 p orbital for the electrons in the double bond, so its lone pair of electrons are not π electrons, there are 6 π electrons, n=1
- Aromatic - there are 6 π electrons, n=1
- Not aromatic - all atoms are sp2 hybridized, but only 1 of S's lone pairs counts as π electrons, so there 8 π electrons, n=1.5
- Not aromatic - there are 4 π electrons, n=1/2
- Aromatic - only 1 of N's lone pairs counts as π electrons, so there are 6 π electrons, n=1
- Not aromatic - not fully conjugated, top C is sp3 hybridized
- Aromatic - O is using its 1 p orbital for the elections in the double bond, so its lone pair of electrons are not π electrons, there are 6 π electrons, n=1
Exercise (PageIndex{2})
Sp2 Hybridized Carbon Ir
To be aromatic, a molecule must be planar conjugated, and obey the 4n+2 rule. The following is the following molecule aromatic?
No, it is not. It does not obey the 4n+2 rule. Also it is not planar.
References
- Vollhardt, Peter, and Neil E. Schore. Organic Chemistry: Structure and Function. 5th ed. New York: W. H. Freeman & Company, 2007.
- Berson, Jerome. Chemical Creativity: Ideas from the Work of Woodward, Hückel, Meerwein, and Others. New York: Wiley-VCH, 1999.
- Badger, G.M. Aromatic Character and Aromaticity. London, England: Cambridge University Press, 1969.
- Lewis, David and David Peters. Facts and Theories of Aromaticity. London, England: Macmillan Press, 1975.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Layne Morsch (University of Illinois Springfield)
- bon and Geoff Hutchison from Chemistry StackExchange
- James Kabrhel (University of Wisconsin - Green Bay, Sheboygan)
