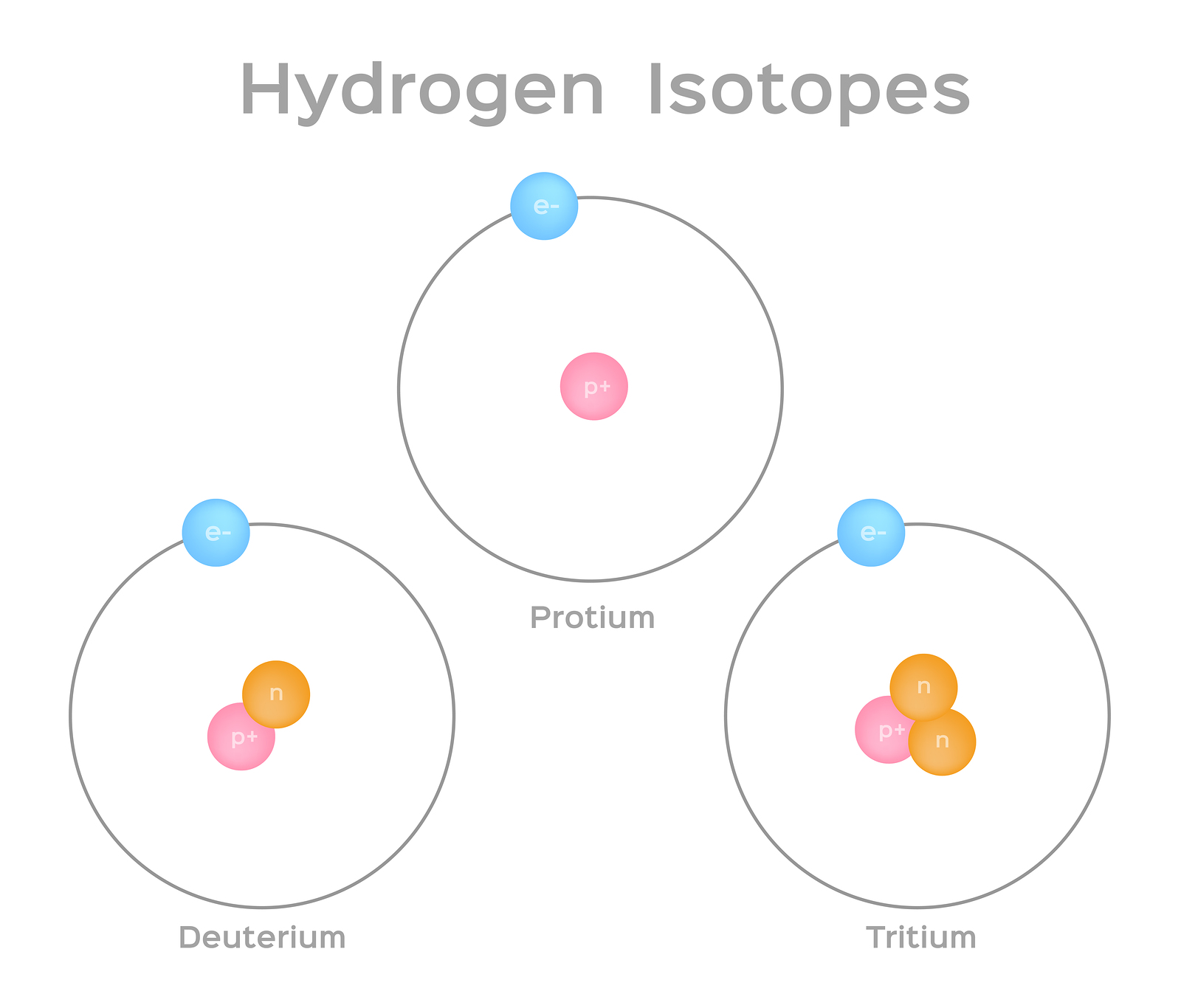
- Atoms of the same element that contain the same number of protons, but different numbers of neutrons, are known as isotopes. Isotopes of any given element all contain the same number of protons, so they have the same atomic number (for example, the atomic number of helium is always 2).
- (a) isotopes as atoms of the same element with different numbers of neutrons and different masses What does this mean? Hopefully, you recall from previous years that Isotopes are different versions.

Isotopes of the same element have the same number of protons and electrons when in neutral atomic form. Different isotopes have different numbers of neutrons in their nuclei, resulting in different atomic weights for the different isotopes of a single element. Isotopes are: atoms of the same element that have the same number of neutrons. Google sync for mac. Atoms of the same element that have different number of protons. Atoms of the same element that have different number of electrons. Atoms of the same element that have different number of neutrons. None of the above. The answer is C) C and D because isotopes are atoms of the same element with different numbers of neutrons. The number of neutrons affects the atomic mass, therefore it is neutrons and atomic mass.

Transcript
Isotopes Of All Elements
Back in the early 1900s, the discoverer of the electron, JJ Thompson with the help of his student Francis W. Aston, identified two different types of neon atoms-- one type with 10 protons, 10 electrons, and 10 neutrons and then one with 10 protons, 10 electrons, and 12 neutrons.
Aston went on to invent a groundbreaking machine that he called a mass spectrograph. Using this machine, Aston identified hundreds of other isotopes of other elements. The mass spectrometer is now used in labs all over the world. And Aston won the 1922 Nobel Prize in chemistry for his work.
These are the isotopes of neon that Thompson and Aston saw. The numbers you see here tell you the total number of protons plus neutrons in each atom. And they also tell you the isotopes approximate mass. Technically, the mass of an atom would include protons, neutrons, and electrons. But since the mass of an electron is almost 2,000 times less than that of protons and neutrons, we can basically ignore them.
When it comes to atoms and their behavior, there's an important distinction to make between chemical reactions and nuclear reactions. Chemical reactivity is defined by the electrons that atoms possess. When atoms come into contact with one another, it's their outer electrons that interact and determine the type and the intensity of the reaction.
Since isotopes of a given element had the same number of electrons, they will undergo similar reactions. On the other hand, nuclear reactions rely on the particles in the nucleus. So different isotopes will undergo different nuclear reactions because of the differences in the makeup of their nuclei.
So what happens in a nuclear reaction? Basically, something changes in the nucleus. Because the nucleus is made up of protons and neutrons, you might think that all of those positive charges crammed together in repelling one another would make the nucleus unstable. Luckily, there's a force known as the strong nuclear force that acts as the glue that holds the whole thing together.
But when the nucleus of an atom contains lots of protons and neutrons or when certain neutron proton ratios exist, even the strong nuclear force can't hold it all together, and the whole thing can become unstable. When that happens, the atoms nucleus will either kick out some particles or rearrange itself.
Typically, one of three things happens. Alpha decay is when two neutrons and two protons are kicked out of the nucleus to form two different species-- a helium nucleus and a totally different element than the one we started with. In this case, uranium ejects the alpha particle and becomes thorium.
In beta decay, a neutron splits into a proton and an electron, and the electron is ejected from the nucleus. Because this changes the number of protons in the nucleus, a new element is formed. In this case, thorium becomes protactinium. Gamma decay is when protons and neutrons in the nucleus are rearranged to reduce the energy of an unstable atom. These rearrangements come with the release of energy in the form of gamma rays. This time, since the number of protons in the nucleus stays the same, no new element is formed.
These spontaneous changes in nuclei are known as radioactivity. While radioactive substances can be hazardous, in some cases, they can also be incredibly useful. They give us an accurate way to tell how old things are. They can be used to detect leaks in pipes or even see where certain medicines go in your body. Thanks for watching. And make sure to check out the other videos in this series here.
[MUSIC PLAYING]